Diffusion experiments
From NMR Wiki
Contents |
Implementations
- Bppste.c (Varian, Inc.)
- Oneshot for Bruker (G.A. Morris/M. Nilsson)
- Oneshot for Varian (G.A. Morris/M. Nilsson)
- Pure shift Oneshot for Varian (G.A. Morris/M. Nilsson)
- Water sLED pl.c (Pascale Legault)
NMR spectroscopy allows measurement of the coefficient of self-diffusion (Ds) which is defined[1] as the translational diffusion coefficient at zero gradient of the chemical potential. Most frequently that condition simply implies zero concentration gradient.
Ds is related to the hydrodynamic properties and size of the molecule indicative of its aggregation state. Unlike ultracentrifugation or dynamic light scattering, both also measuring Ds, NMR methods work at mM concentration, i.e. in the conditions used for structural work by NMR.
LED
LED stands for longitudinal encode-decode or "longitudinal eddy current delay" experiment. The LED experiment is an evolutionary improvement of the PFGSTE (stimulated echo: 90o-τPFG-90o-T-90o-τPFG-acquire) experiment proposed by Tanner in 1970[2]. In LED experiments[3] magnetization is stored along the z-axis during most of the pulse sequence, so T1 relaxation is predominant. Since in macromolecules the T1 relaxation is slower than the T2 relaxation, the LED experiment is better suited to the measurement of Ds of slower diffusing molecules where longer "diffusion delay" is required to detect attenuation of the signal.
The water-sLED experiment[4] is a modification of the LED experiment[3] with added water suppression (Water flip-back and RAW). water-sLED allows determination of Ds for molecules with molecular weights of up to ~40 kDa.
The amplitude of the echo in these experiments follows the equation below:
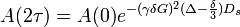
Where:
- γ - gyromagnetic ratio of the nucleus
- δ - duration of the PFG
- G - gradient strength
- Ds - constant of self-diffusion
- Δ time between beginnings of the two gradient pulses
BPP-SED
The BPP-SED experiment [5] combines the BPP-LED and SED (selective echo dephasing) water suppression schemes; it is designed to measure self-diffusion constants of biomolecules in up to 95% H2O. This experiment requires a tri-axial PFG probe as gradients along the x,y,z axes are used.
References
- IUPAC definition of self-diffusion coefficient
-
Tanner, JE. Use of the Stimulated Echo in NMR Diffusion Studies. Journal of Chemical Physics 52:2523--2526, 1970. BibTeX
-
GIBBS, SJ and JOHNSON, CS. A PFG NMR experiment for accurate diffusion and flow studies in the presence of Eddy currents. Journal of magnetic resonance 93(2):395--402, 1991. BibTeX
-
Altieri, AS and Hinton, DP and Byrd, RA. Association of Biomolecular Systems via Pulsed Field Gradient NMR Self-Diffusion Measurements. Journal of the American Chemical Society 117(28):7566--7567, 1995. BibTeX
-
Krishnan, VV and Thornton, KH and Cosman, M. An improved experimental scheme to measure self-diffusion coefficients of biomolecules with an advantageous use of radiation damping. Chemical Physics Letters 302(3-4):317--323, 1999. BibTeX
-
Hahn, EL. Spin Echoes. Physical Review 80(4):580--594, 1950. BibTeX
- Lecture on DOSY by Prof. Stéphane Viel
-
STILBS, P. Fourier transform pulsed-gradient spin-echo studies of molecular diffusion. Progress in nuclear magnetic resonance spectroscopy 19(1):1-45, 1987. BibTeX
-
Price, William S. Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion: Part 1. Basic theory. Concepts in Magnetic Resonance 9(5):299-336, 1997. BibTeX | Abstract
-
Price, William S. Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion: Part II. Experimental aspects. Concepts in Magnetic Resonance 10(4):197-237, 1998. BibTeX
-
Johnson, Jr , Charles S. Diffusion ordered nuclear magnetic resonance spectroscopy: principles and applications. Progress in Nuclear Magnetic Resonance Spectroscopy 34:203-256, 1999. BibTeX
-
Antalek, Brian. Using pulsed gradient spin echo NMR for chemical mixture analysis: How to obtain optimum results. Concepts in Magnetic Resonance 14(4):225-258, 2002. BibTeX | Abstract
-
Morris, Gareth A. Diffusion-Ordered NMR Spectroscopy. , Chichester, 2002. BibTeX
-
Armstrong, Geoffrey S and Loening, Nikolaus M and Curtis, Joseph E and Shaka, A J and Mandelshtam, Vladimir A. Processing DOSY spectra using the regularized resolvent transform. Journal of Magnetic Resonance 163(1):139-148, 2003. BibTeX
-
Viel, Stephane and Ziarelli, Fabio and Caldarelli, Stefano. Enhanced diffusion-edited NMR spectroscopy of mixtures using chromatographic stationary phases. PNAS 100(17):9696-9698, 2003. BibTeX | Abstract
-
Cohen, Yoram and Avram, Liat and Frish, Limor. Diffusion NMR Spectroscopy in Supramolecular and Combinatorial Chemistry: An Old Parameter-New Insights. Angewandte Chemie International Edition 44(4):520-540, 2005. BibTeX | Abstract
-
Brand, Torsten and Cabrita, Eurico J and Berger, Stefan. Intermolecular interaction as investigated by NOE and diffusion studies. Progress in Nuclear Magnetic Resonance Spectroscopy 46:159-196, 2005. BibTeX
-
Thureau, P and Ancian, B and Viel, S and Thevand, A. Determining chemical exchange rates of the uracil labile protons by NMR diffusion experiments. Chemical Communications , 2006. BibTeX | Abstract
-
Viel, Stephane and Ziarelli, Fabio and Pages, Guilhem and Carrara, Caroline and Caldarelli, Stefano. Pulsed field gradient magic angle spinning NMR self-diffusion measurements in liquids. Journal of Magnetic Resonance 190(1):113-123, 2008. BibTeX | Abstract

