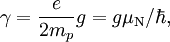Gyromagnetic ratio
From NMR Wiki
(Difference between revisions)
| Line 19: | Line 19: | ||
|- | |- | ||
! species | ! species | ||
| - | ! <i>γ / 2π</i> (MHz/T) | + | ! <i>γ / 2π</i> (MHz/T) <cite>nistval</cite> |
|- | |- | ||
|free electron | |free electron | ||
| - | | | + | |2.80249536 x 10<sup>4</sup> <cite>niste</cite> |
|- | |- | ||
| <sup>1</sup>H | | <sup>1</sup>H | ||
| Line 74: | Line 74: | ||
==References== | ==References== | ||
<biblio> | <biblio> | ||
| + | #nistval values are divided by 2*pi, those in nist tables are not | ||
#niste [http://physics.nist.gov/cgi-bin/cuu/Value?qgammae|search_for=gyromagnetic+ratio+electron gyromagnetic ratio of free electron] | #niste [http://physics.nist.gov/cgi-bin/cuu/Value?qgammae|search_for=gyromagnetic+ratio+electron gyromagnetic ratio of free electron] | ||
</biblio> | </biblio> | ||
Revision as of 21:52, 22 April 2009
Gyromagnetic Ratio is defined by:
where 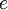 is the magnitude of electron charge,
is the magnitude of electron charge, 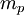 is the proton mass,
is the proton mass, 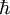 is reduced Planck constant,
is reduced Planck constant, 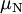 is the nuclear magneton, and
is the nuclear magneton, and 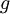 is the g-factor of the nucleon or nucleus in question.
is the g-factor of the nucleon or nucleus in question.
Approximate values for some common nuclei are given in the Table below.<ref>Template:Cite book</ref>
| species | γ / 2π (MHz/T) [1] | |
|---|---|---|
| free electron | 2.80249536 x 104 [2] | |
| 1H | 42.576 | |
| 2D | 6.53593 | |
| 3He | -32.434 | |
| 7Li | 16.546 | |
| 13C | 10.705 | |
| 14N | 3.0766 | |
| 15N | -4.3156 | |
| 17O | -5.7716 | |
| 19F | 40.0593 | |
| 23Na | 11.262 | |
| 31P | 17.235 | |
| 75As | 7.2919 | |
| 129Xe | -11.777 |
For information on individual chemical elements please use NMR and EPR Periodic Table
To find NMR frequency of your favorite nucleus at a given magnetic field, see Alex Jerschow's on-line Interactive NMR Frequency Map
References
- values are divided by 2*pi, those in nist tables are not
- gyromagnetic ratio of free electron